Menu
Are you ready for the next era of precision surgery? Say hello to Auxein’s Patient-Specific Implants (PSI)!
A Patient-Specific Implant (PSI) is a customized medical implant designed to match the exact anatomy of an individual patient. Using advanced 3D imaging, CAD design, and precision manufacturing, PSI ensures better anatomical fit, enhanced function, and faster recovery compared to conventional implants.
Using advanced imaging (like CT scans) and cutting-edge 3D modelling and manufacturing (CAD/CAM and 3D printing), Auxein creates a perfect replica of the missing or damaged bone structure. This ensures a perfect, anatomical fit that matches your unique bone structure.
Auxein PSI’s precision is vital in surgeries involving complex, delicate, and visible bone structures. They are primarily used in:
1. CranioMaxillofacial Surgery (CMF):

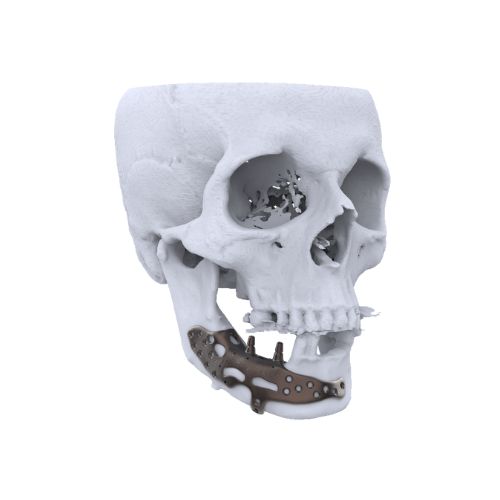
2. Neuro Surgery:
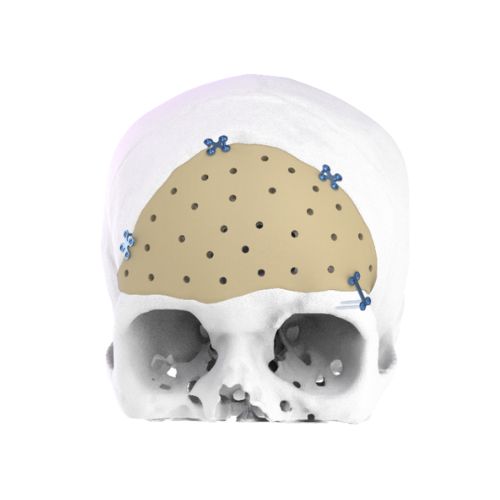

3. Onco Surgery (Oncological Reconstruction):
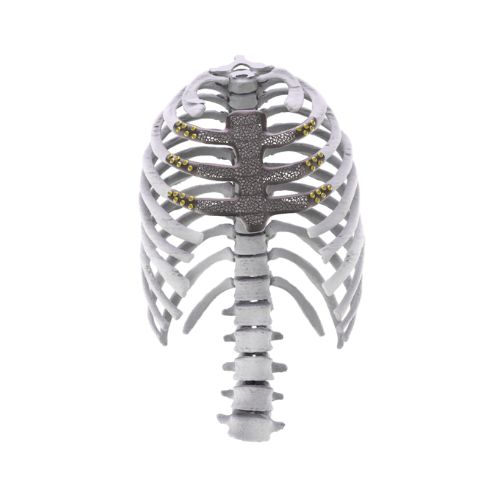
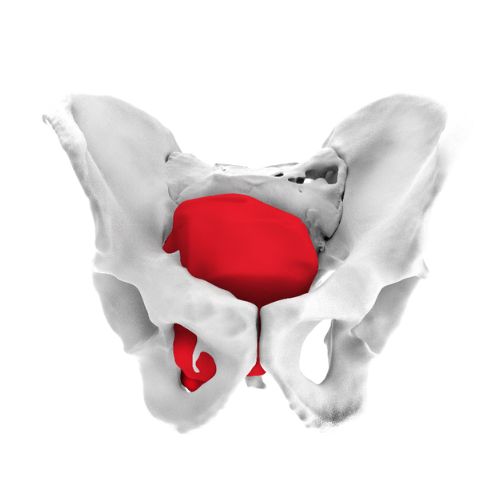
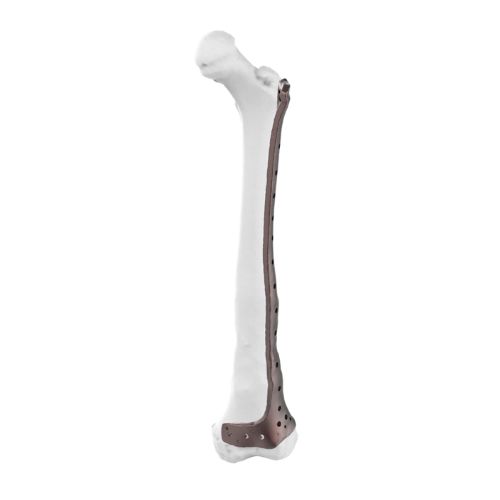
Auxein doesn’t just make custom implants; it pioneers the technology to make them better, stronger, and more precise than ever before.
Comparison: PSI vs Standard Stock Implant
Feature | Patient-Specific Implants (PSI) | Standard Stock / Preformed Implants |
Fit & Accuracy | Exact anatomical fit (mirrored from healthy side); minimal intra-op modification needed. | Require intraoperative bending/shaping; may not fit complex defects precisely. |
Surgical Time | Reduced (shorter operation due to precise fit). | Longer (time consumed in adjusting implant during surgery). |
Aesthetic & Functional Outcome | Superior contouring and symmetry; high patient satisfaction. | Often suboptimal results due to less precise adaptation. |
Complications | Very low incidence of infection, displacement, or resorption (none reported in this study). | Higher risk of infection, resorption, displacement, and donor site morbidity (in autografts). |
Customization | Designed for each patient using 3D planning and AM. | Generic, one-size-fits-most designs; limited adaptability in complex cases. |
Materials | PEEK, UHMWPE, Titanium (biocompatible, durable, radiolucent options available). | Usually titanium mesh, silicone, porous polyethylene, or standard plates. |
Auxein PSI: Beyond mass-produced, beyond standard. It’s the future of personalized surgery, delivering unparalleled fit, strength, and confidence to patients globally.
Reference:
US FDA 510k Cleared, CE Marked, MDSAP, ISO 13485:2016, EU-MDR 2017 certified
Indian FDA Approved | GMP Certificate
USA
Auxein Inc.
1500 Nw 89th Court, Suite 107-108,
Doral, Florida 33172
Tel: +1 305 395 6062
E Fax: +1 305 395 6262
Email: USoffice@auxein.com
Mexico
Auxein México S.A. de C.V.
Tepic 139 int 801, Colonia
Roma Sur,
Alcaldía Cuauhtémoc, CDMX,
México, C.P. 06760
Tel: +521 55 7261 0318
Email: info@auxein.mx
Dubai UAE
Auxein Medical Trading FZCO
Oud Metha Offices Building,
B Block 1st Floor Office No.26,
Near Healthcare City,
PO BOX-25572
Tel: +971 438 71333
Email: info@auxein.com
India
Auxein Medical Pvt. Ltd.
Plot No. 168-169-170, Phase-4,
Kundli Industrial Area,
HSIIDC, Sector-57, Sonepat – 131028,
Haryana
Tel: +91 99106 43638 | Fax: +91 86077 70197
Email: info@auxein.com