Menu
Anterior Cruciate Ligament (ACL) reconstruction remains one of the most performed procedures in sports medicine. While graft selection has traditionally revolved around Hamstring Tendon (HT), Bone–Patellar Tendon–Bone (BTB), and Quadriceps Tendon (QT), emerging research in recent years has introduced the Peroneus Longus Tendon (PLT) as a strong, versatile alternative. As surgical outcomes continue to evolve, so does the evidence guiding graft choice — making 2026 an ideal moment to revisit the data.
Each traditional graft type carries unique strengths and limitations:
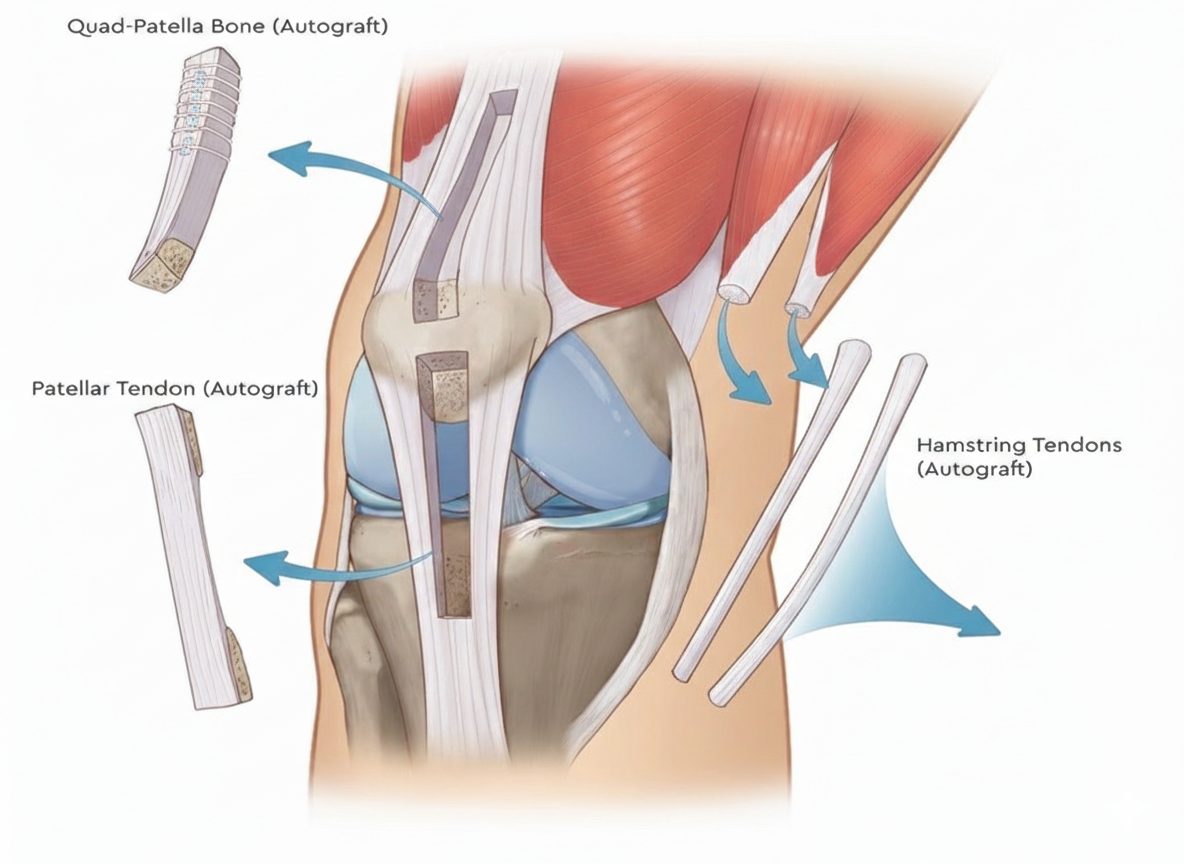
Recent literature has positioned the Peroneus Longus Tendon as a viable graft with strength and functionality comparable to BTB and HT grafts. Its key advantages include minimal donor-site morbidity, preserved ankle stability (due to peroneus brevis compensation), and excellent graft length and diameter.
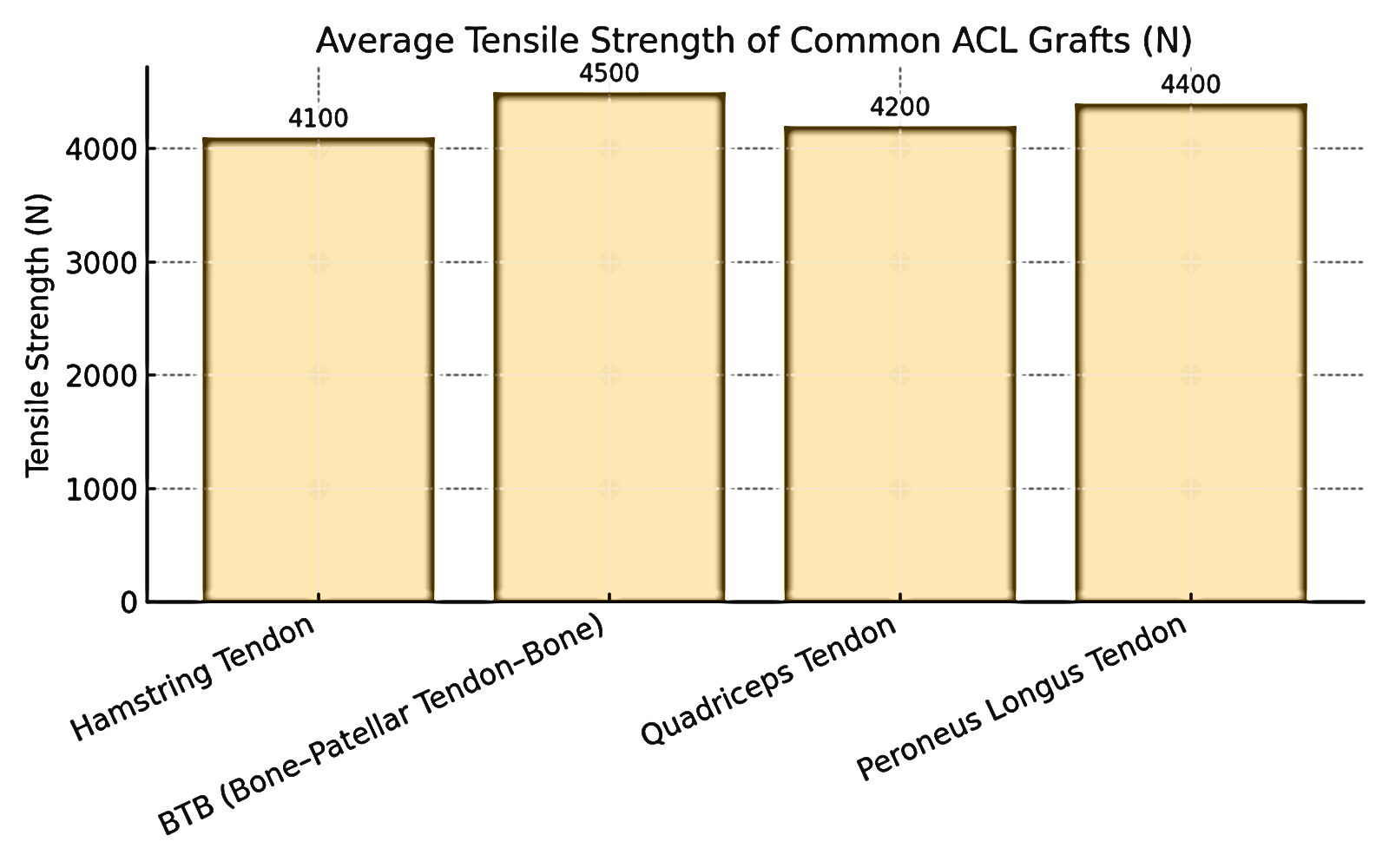
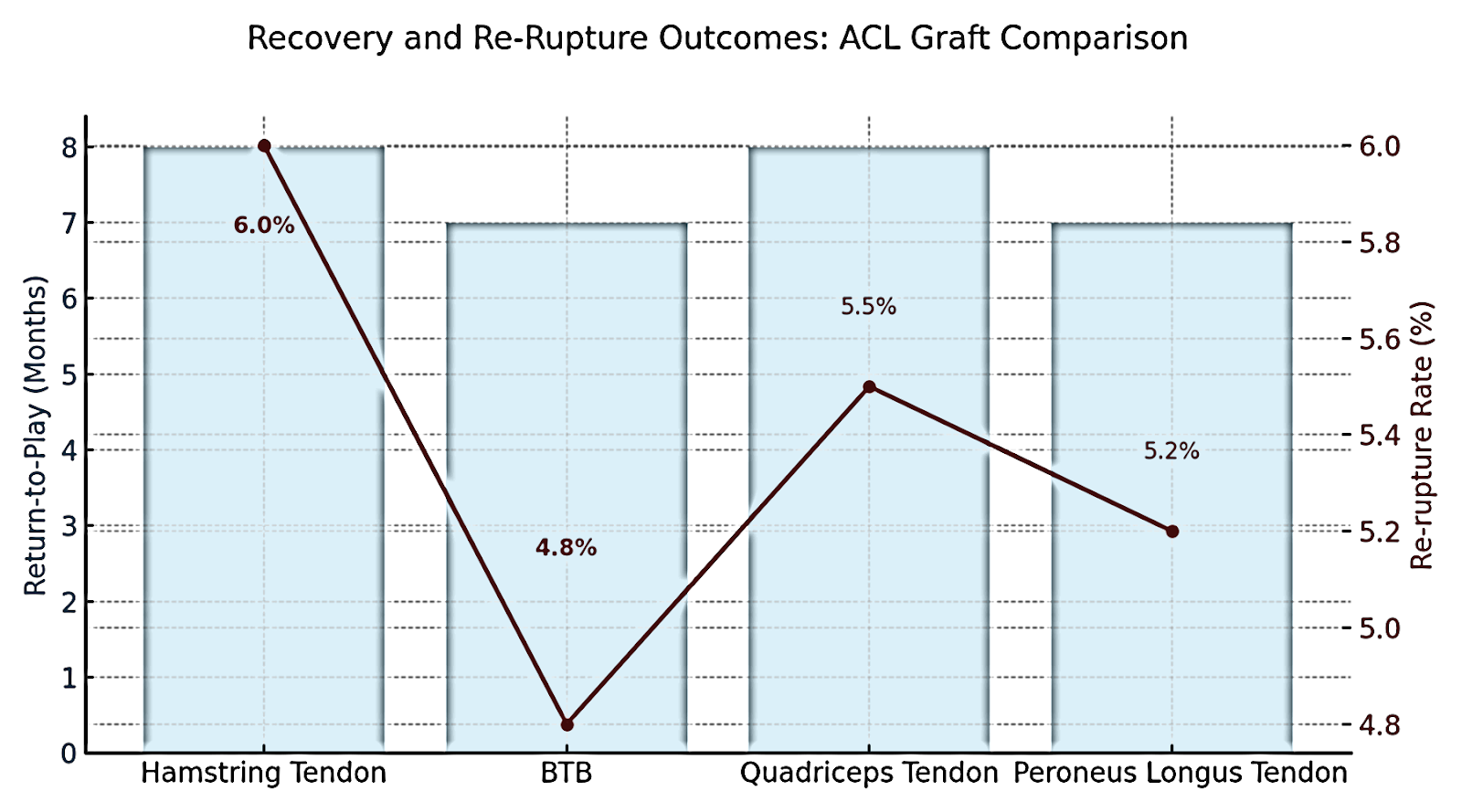
Parameter | Hamstring Tendon (HT) | BTB | Quadriceps Tendon (QT) | Peroneus Longus Tendon (PLT) |
Tensile Strength (N) | 4100 | 4500 | 4200 | 4400 |
Return-to-Play (Months) | 8 | 7 | 8 | 7 |
Re-Rupture Rate (%) | 6.0 | 4.8 | 5.5 | 5.2 |
Donor Site Morbidity | Low | Moderate | Low | Low |
Ideal Candidate | General athlete | High-demand athlete | Revision or large graft | Versatile, low-morbidity case |
At Auxein, we believe that graft performance is not merely about tensile strength, but also about achieving seamless biological integration. The synergy between optimal graft choice and reliable fixation technology determines the long-term success of ACL reconstructions.
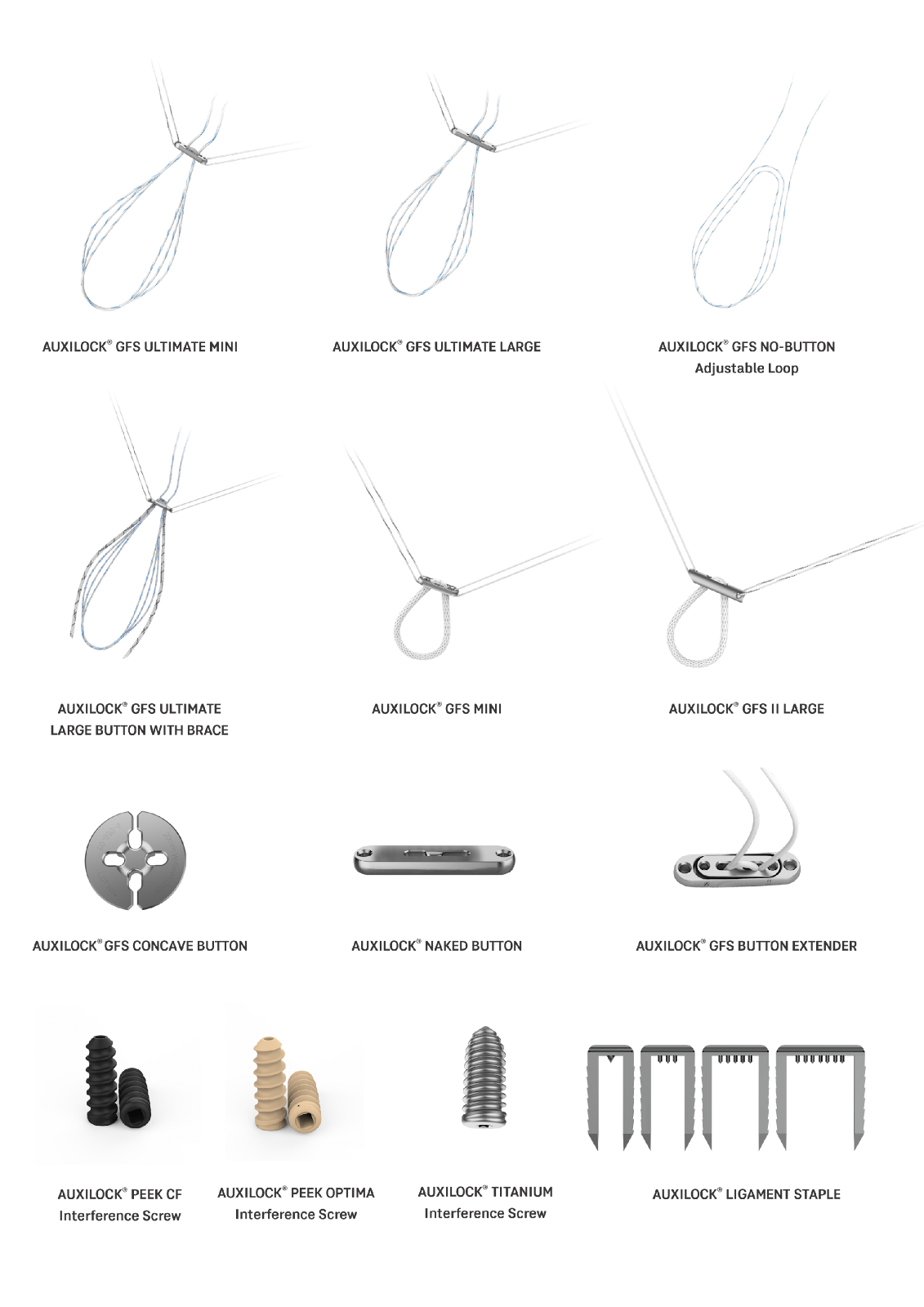
Auxein’s AUXILOCK® ACL Fixation Range provides surgeons with a comprehensive range of adjustable-loop and fixed-loop options, concave buttons, naked buttons, interference screws, and supportive ligament staples — engineered for precise tensioning, secure fixation, and reliable performance.
In 2026, the landscape of ACL reconstruction continues to evolve toward personalized, evidence-based graft selection. The Peroneus Longus Tendon stands out as a promising graft option, combining strong biomechanical performance with low donor-site morbidity. With Auxein’s advanced fixation technology, surgeons are equipped to achieve the dual goal of mechanical stability and biological harmony — enabling athletes to return stronger, faster, and safer.
US FDA 510k Cleared, CE Marked, MDSAP, ISO 13485:2016, EU-MDR 2017 certified
Indian FDA Approved | GMP Certificate
USA
Auxein Inc.
1500 Nw 89th Court, Suite 107-108,
Doral, Florida 33172
Tel: +1 305 395 6062
E Fax: +1 305 395 6262
Email: USoffice@auxein.com
Mexico
Auxein México S.A. de C.V.
Tepic 139 int 801, Colonia
Roma Sur,
Alcaldía Cuauhtémoc, CDMX,
México, C.P. 06760
Tel: +521 55 7261 0318
Email: info@auxein.mx
Dubai UAE
Auxein Medical Trading FZCO
Oud Metha Offices Building,
B Block 1st Floor Office No.26,
Near Healthcare City,
PO BOX-25572
Tel: +971 438 71333
Email: info@auxein.com
India
Auxein Medical Pvt. Ltd.
Plot No. 168-169-170, Phase-4,
Kundli Industrial Area,
HSIIDC, Sector-57, Sonepat – 131028,
Haryana
Tel: +91 99106 43638 | Fax: +91 86077 70197
Email: info@auxein.com