Menu
Lateral Extra-Articular Tenodesis (LET) has regained prominence as a crucial complement to Anterior Cruciate Ligament Reconstruction (ACLR), enhancing rotational stability and reducing the rate of graft failure. Integrating biomechanical innovation with clinical research, Auxein continues to redefine fixation strategies for optimal surgical outcomes.
Auxein’s design philosophy merges biomechanical integrity with surgical efficiency. Our anchor systems, fixation devices, and graft management solutions ensure consistent performance and long-term joint stability. By aligning engineering innovation with clinical need, Auxein enables surgeons to restore mobility with confidence.
LET enhances rotational control by resisting excessive tibial internal rotation and anterior translation. Combined with ACLR, it decreases graft strain by approximately 50% (Guenther et al., JBJS, 2017), ensuring a balanced load-sharing mechanism. Studies consistently show that the dual approach reduces pivot shift phenomena and lowers graft rupture rates.
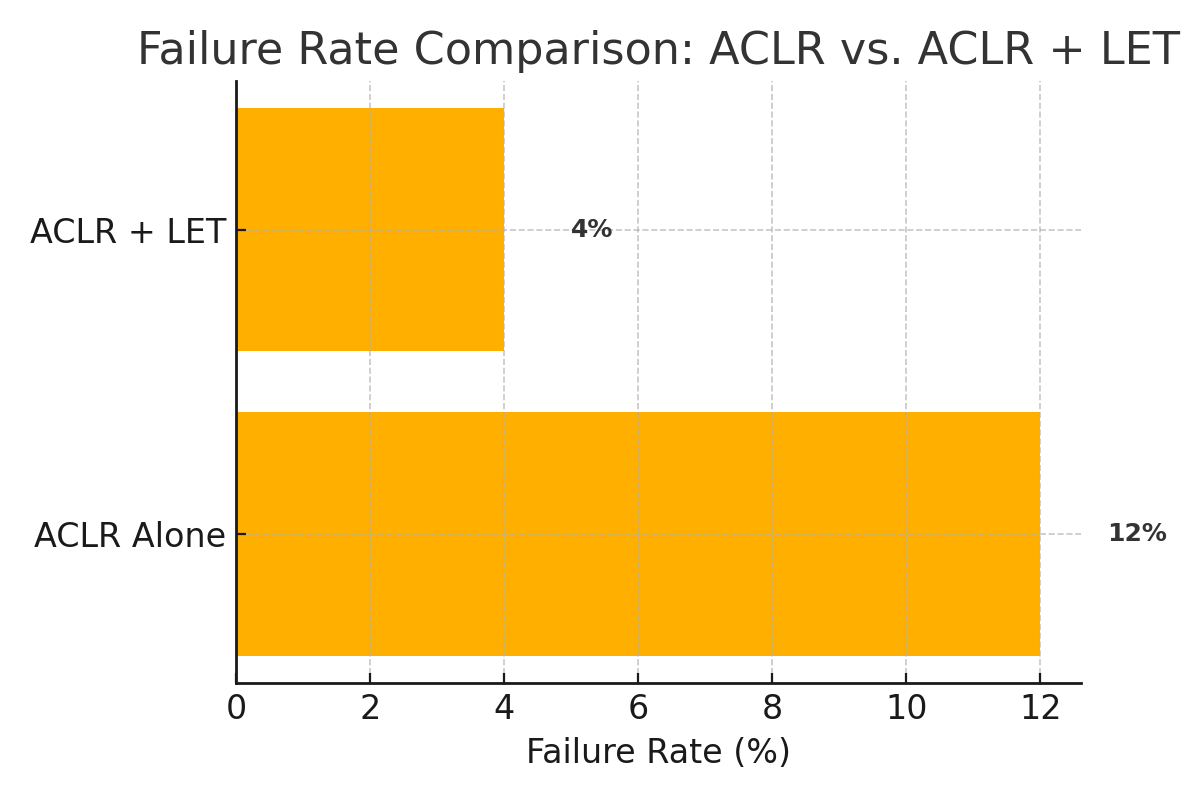
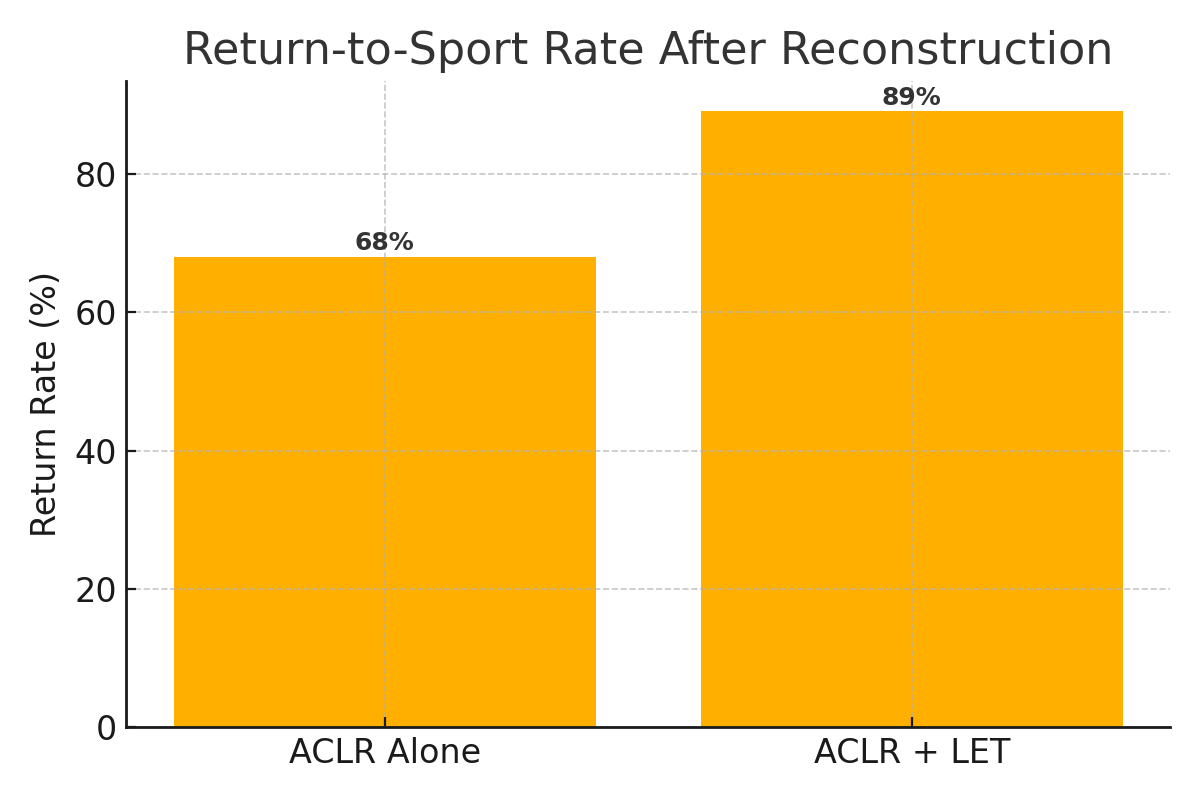
| Study (Year) | Procedure | Failure Rate | Pivot Shift Reduction | Patient Satisfaction |
| Rowan et al., 2019 | ACLR + LET | ↓3.8% | ↓45% | 92% |
| Perelli et al., 2023 | Isolated LET | N/A | Moderate | 85% |
| Minguell et al., 2023 | Revision ACLR + LET | ↓5–10% | Strong | 89% |
| Zanna et al., 2023 | ACLR + LET | ↓35% | ↓60% | 94% |
| Condello et al., 2019 | Allograft ACLR + LET | ↓40% strain | ↓48% | 90% |
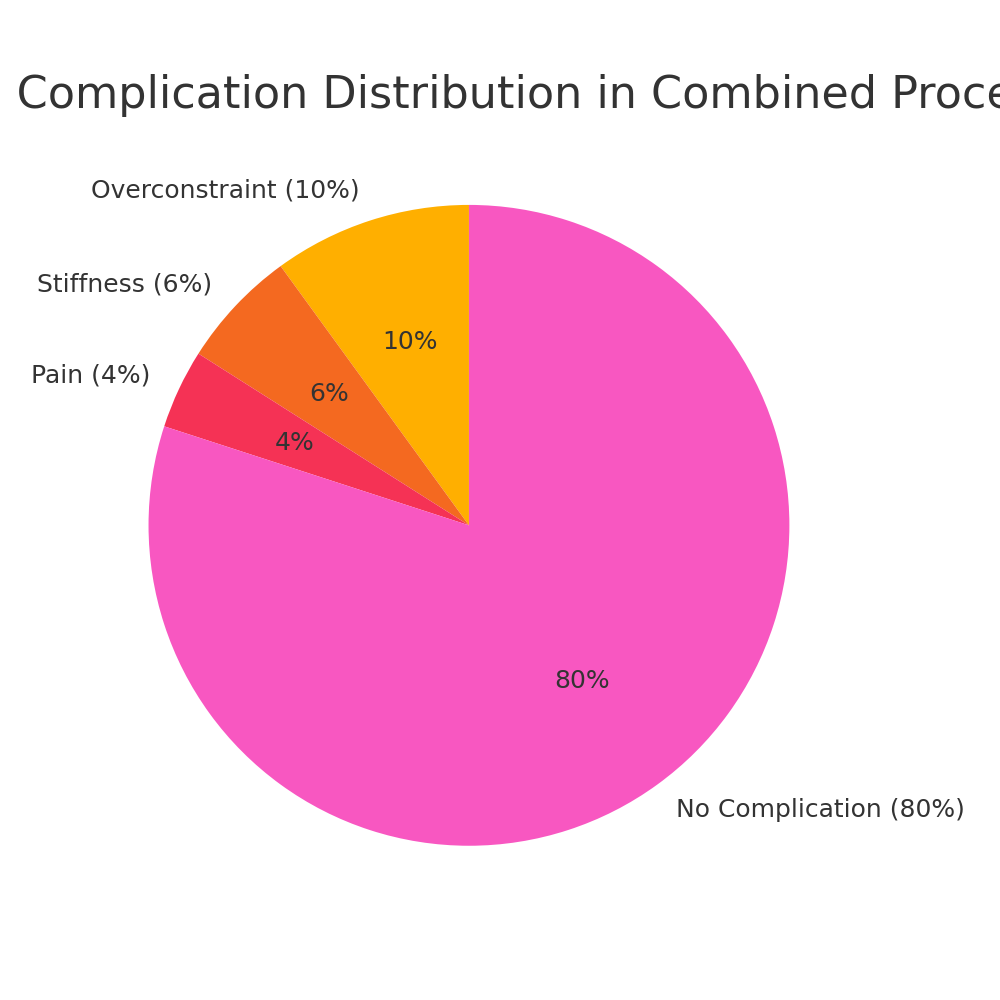
Indication | Rationale | Preferred Technique |
High-grade pivot shift | Restores anterolateral restraint | Modified Lemaire LET |
Revision ACLR | Reduces re-rupture risk | Modified MacIntosh |
Generalized laxity | Controls tibial internal rotation | Iliotibial Band LET |
High-demand athletes | Prevents graft overload | Hamstring + LET |
Lateral Extra-Articular Tenodesis combined with ACL Reconstruction represents the next step in restoring native knee biomechanics. By reducing rotational laxity and enhancing joint stability, this dual reconstruction ensures longevity and performance. Through continued innovation, simulation, and clinical partnership, Auxein is shaping the future of orthopedic biomechanics.
US FDA 510k Cleared, CE Marked, MDSAP, ISO 13485:2016, EU-MDR 2017 certified
Indian FDA Approved | GMP Certificate
USA
Auxein Inc.
1500 Nw 89th Court, Suite 107-108,
Doral, Florida 33172
Tel: +1 305 395 6062
E Fax: +1 305 395 6262
Email: USoffice@auxein.com
Mexico
Auxein México S.A. de C.V.
Tepic 139 int 801, Colonia
Roma Sur,
Alcaldía Cuauhtémoc, CDMX,
México, C.P. 06760
Tel: +521 55 7261 0318
Email: info@auxein.mx
Dubai UAE
Auxein Medical Trading FZCO
Oud Metha Offices Building,
B Block 1st Floor Office No.26,
Near Healthcare City,
PO BOX-25572
Tel: +971 438 71333
Email: info@auxein.com
India
Auxein Medical Pvt. Ltd.
Plot No. 168-169-170, Phase-4,
Kundli Industrial Area,
HSIIDC, Sector-57, Sonepat – 131028,
Haryana
Tel: +91 99106 43638 | Fax: +91 86077 70197
Email: info@auxein.com