Menu
Orthopedic fixation has evolved beyond mechanical stabilization to become a biomechanically intelligent process. At Auxein Medical, our approach to implant design merges scientific precision with the body’s natural healing. Our fixation systems redefine how fractures heal — not just stabilized, but optimized.
At Auxein, we engineer implants that respect biology. Each system — plate, screw, or intramedullary nail — is designed to ensure robust fixation while preserving vascularity. Through collaboration with global orthopedic leaders, Auxein continues to innovate for clinical excellence and patient recovery.
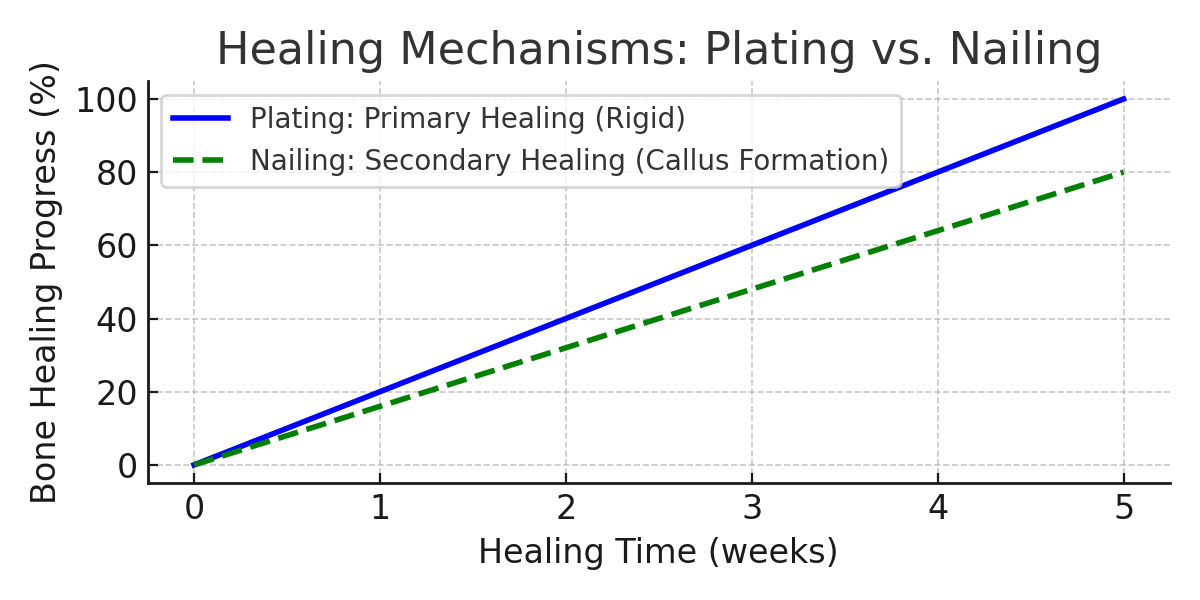
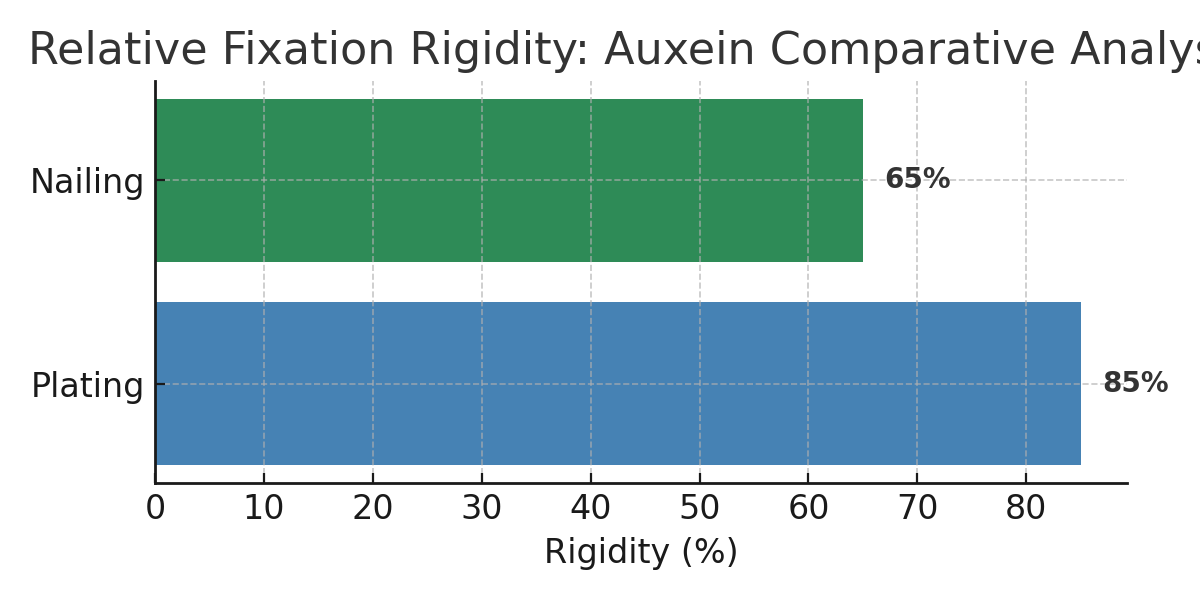
Both plating and nailing have distinct biomechanical properties. Plating provides rigid fixation and anatomic precision, while nailing supports elastic fixation and callus-driven healing. The optimal choice depends on fracture pattern, bone quality, and surgeon preference.
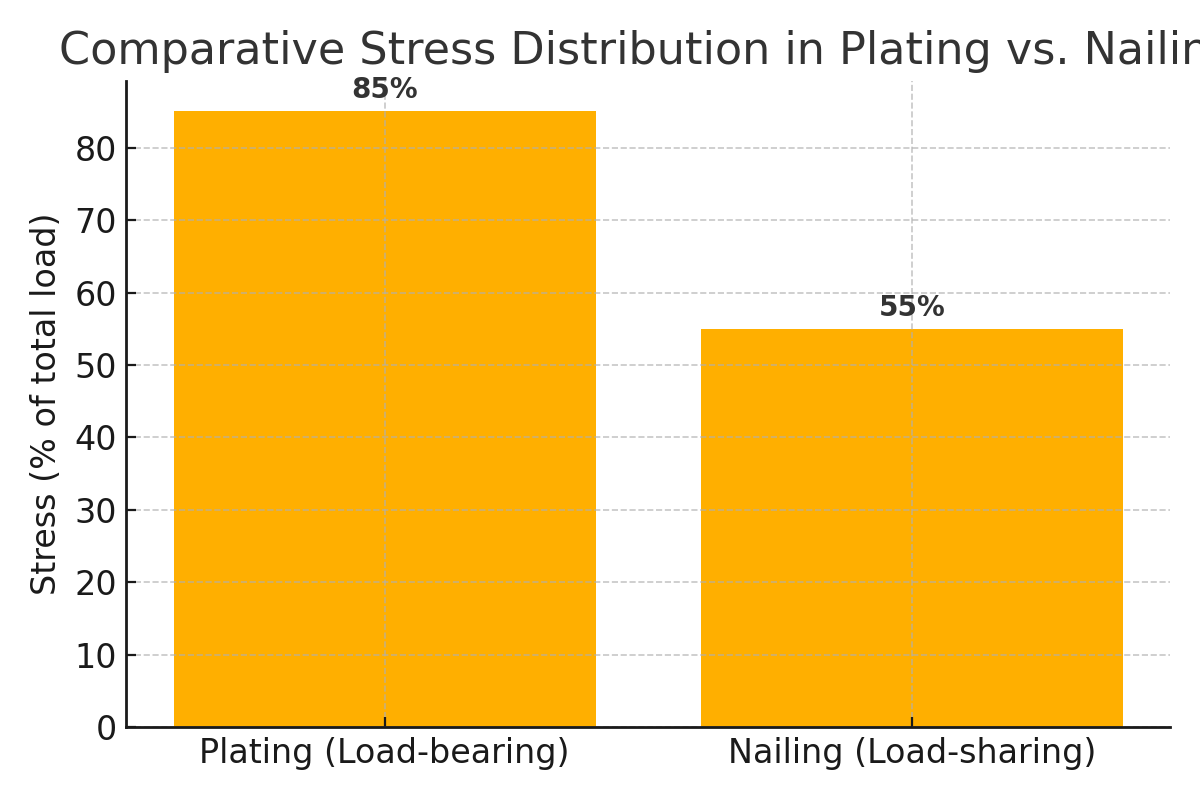
The Auxein Blend philosophy merges mechanical strength with biological adaptability. Our hybrid fixation constructs combine the rigidity of plating with the micromotion benefits of nailing — providing adaptive stability that encourages faster, more physiological healing.
Every Auxein product is surgeon-informed and patient-focused. Ergonomic instrumentation, pre-contoured designs, and intuitive targeting systems empower precision and reduce operative time. Our devices ensure clinical confidence and reproducibility in the OR.
Auxein integrates advanced material science, 3D printing, and biomechanical modeling into its R&D workflow. Finite Element Analysis (FEA) simulations guide design optimization, ensuring performance even under complex stress conditions. Every implant meets rigorous ISO, CE, and FDA criteria for global reliability.
| Fracture Type | Preferred Method | Clinical Advantage | Reference |
| Proximal Tibia | Plate (MIPO) | Superior angular control | Dragonas et al., 2025 |
| Shaft Humerus | Nail | Minimal incision, early mobility | Wali et al., 2014 |
| Distal Femur | Hybrid (Plate + Nail) | Enhanced torsional stability | Anaspure et al., 2024 |
| Proximal Femur | Nail | Rapid weight-bearing recovery | Mert et al., 2025 |
| Proximal Humerus | Locking Plate | Anatomical reduction precision | Guo et al., 2022 |
Auxein’s facilities embody green manufacturing principles — from energy-efficient production to recyclable packaging. Each implant undergoes in-house testing, ensuring durability, traceability, and minimal environmental footprint.
Plating delivers precision; nailing delivers biology. Auxein delivers both. By merging biomechanical science with surgical insight, we redefine the standards of fixation technology — ensuring every fracture heals not only faster but smarter.
US FDA 510k Cleared, CE Marked, MDSAP, ISO 13485:2016, EU-MDR 2017 certified
Indian FDA Approved | GMP Certificate
USA
Auxein Inc.
1500 Nw 89th Court, Suite 107-108,
Doral, Florida 33172
Tel: +1 305 395 6062
E Fax: +1 305 395 6262
Email: USoffice@auxein.com
Mexico
Auxein México S.A. de C.V.
Tepic 139 int 801, Colonia
Roma Sur,
Alcaldía Cuauhtémoc, CDMX,
México, C.P. 06760
Tel: +521 55 7261 0318
Email: info@auxein.mx
Dubai UAE
Auxein Medical Trading FZCO
Oud Metha Offices Building,
B Block 1st Floor Office No.26,
Near Healthcare City,
PO BOX-25572
Tel: +971 438 71333
Email: info@auxein.com
India
Auxein Medical Pvt. Ltd.
Plot No. 168-169-170, Phase-4,
Kundli Industrial Area,
HSIIDC, Sector-57, Sonepat – 131028,
Haryana
Tel: +91 99106 43638 | Fax: +91 86077 70197
Email: info@auxein.com